This web page was produced as an assignment for Genetics 677, an undergraduate course in UW Madison
Popular press review
In the article “Loss of the protein target of lithium disrupts normal mouse embryonic heart development”(2008, October 8, http://www.sciencedaily.com )the authors start to discuss the controversies in using lithium for treatment of bipolar disorder. According to the article there are some suggested links between congenital heart defects and lithium, which is a GSK3 inhibitor, however it also states that new medications which are even more powerful GSK3 inhibitors are subject of new research.
The authors further quote a study on mice done by scientists from Thomas Jefferson University in Philadelphia who report that mice embryos, lacking GSK3-beta, died before birth – mostly during the late developmental stages. The defects observed from these embryos affected the heart and some of them included thickening of the cardiac muscle as a result from excessive proliferation of the cells. In contrast mice which were deficient of GSK3-alfa were born with normal hearts.
In conclusion the authors suggest that lithium and other new generation GSK3 inhibitors need to be carefully considered before prescribed for treatment to women of childbearing age although the controversy about the teratogenic effects of lithium remains.
The reason I chose this article is the interesting question that it raises – the possible role of GSK3-beta in embryonic development and especially in heart muscle development. The scientific research quoted by the authors provides strong evidence that the enzyme have a significant effect in this process. Although the text mentions lithium as an example of treatment for bipolar disorder only other resources [8] suggest that new GSK-3 inhibitors can find potential use in treatment for conditions like diabetes, Alzheimer disease and even stroke. Considering the fact that GSK3 is a key component in crucial biological pathways such as the Wnt/beta-catenin pathway I agree with the authors that these new treatment drugs will need to be cautiously addressed.
| deletion_of_gsk-3b_in_mice_leads_to_hypertrophic_cardiomyopathy_secondary_to_cardiomyoblast_heperproliferation..pdf | |
| File Size: | 2473 kb |
| File Type: | |
| supplimentary_figures.pdf | |
| File Size: | 1705 kb |
| File Type: | |
Scientific article review.
Inhibitors for Gsk-3 have been widely used for treatment of bipolar disorders. However there have been some contradictory opinions about the involvement of such drugs, especially lithium, in development of congenital heart defects [16]. In order to address the validity of this question the scientists in this paper [2] try to conduct an in-depth research in order to determine the possible role that Gsk3 plays in heart development. They are able to construct a strong comparative analysis between three different types of mice - wild type, ones deficient in Gsk3a (Gsk3a-/-) gene and ones deficient in Gsk3b (Gsk3b-/-) gene. The level of comparison is conducted on both tissue level and embryonic level by comparing phenotypic changes that occur in the cardiac muscle tissue development and the early structural changes that can be observed in the affected organisms. The scientists even further attempt to provide explanation about the molecular mechanism of protein-protein interactions by comparing the differences in gene expression of a discrete number of genes between the different lines. The genes they try to examine are known to be involved in developmental processes as well as specifically in cardiomyocytes proliferation.
One of the most interesting finding in this paper for me was the proposed redundant mechanism by which the two isoforms of Gsk3 control beta-catenin pathway and the fact that the beta-catenin localization remains unaffected in either mutant lines. I found the evidence particularly strong due to the fact that the mutant line completely lacked one of the protein isoforms - the mutant mice were created by a "knock out" mechanism of the gene. Unfortunately as the abstract suggested I expected to find more information about the mechanism of inhibition of this molecule and its regulatory pathway especially addressing the different domains of the kinase that are subject of this complex regulation. Nevertheless the paper concentrated on the phenotypic findings and the conclusions provided from the researcher's findings were strongly supported and can be useful for future studies.
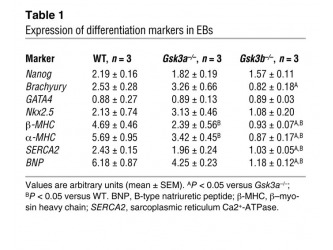
The first step of their experiment was to determine if there is any observed differences between differentiation patterns in wild type ES cell line (WT), and ES cells lacking either the alfa or the beta isoform (Gsk3a-/- and Gsk3b-/- respectively) and to compare expression of gene markers known to participate in cardiomyocytes differentiation (Table 1) between the three lines. The data shows that Nanog, GATA4 and Nkx 2.5, which are markers for early heart development, have similar level of gene expression between the three lines. The markers for late cardiac development aMHC, bMHC, SERCA2 and BNP were significantly reduced in the Gsk3b-/- cell line. The scientists were able to conclude that the Gsk3b is necessary for the late stages of heart development.
Comment: In this initial conclusion though the researchers only acknowledge the fact that level of expression of brachyury (marker for early mesoderm development[2]) is significantly reduced in the Gsk3b-/- line without discussing the possible connection between this marker and the kinase in question. Especially interesting is the fact that this marker is an important component in the FGF signaling pathway [17] which was also reported by Kerkela to be a regulator in cardiomyocytes proliferation. In that matter the data provided by the researchers suggest that Gsk3b is required not only for the late but also the earlier stages of myocyte differentiation.
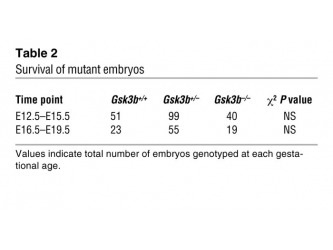
During the second step of the investigation the researchers examined the effect of lack of Gsk3a and Gsk3b isoforms in mice embryos. Their studies showed that mice deficient for Gsk3a were fertile and did not show any cardiac malformations after comparison with the mice Gsk3+/+ ( see Supplementary Figure1) and in contrast they were not able to obtain live Gsk3b-/- mice. In order to examine the possibility that the reason for higher embryonic death was due to liver failure the researchers genotypes 287 embryos ranging from 12.5 embryonic days (E12.5) to 19.5 embryonic days (E19.5). The results summarized in Table 2 show that the number of Gsk3b-/- was not significantly lower than wt and heterozygous embryos. According to the scientists if liver failure was the reason for embryonic death in the mutant line the amount of Gsk3b-/- embryos recovered would have been significantly lower.
The author's attention is now shifted to the possible congenital heart defects that might be the reason for the higher prenatal death rate. After careful comparison the scientists were able to identify the following defects observed in the Gsk3b-/- mice embryos.
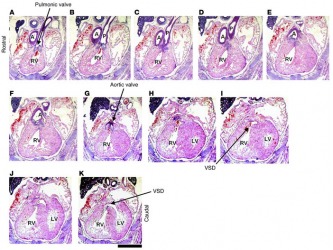
For example they noticed that in knock out Gsk3b mutants both the aorta and the pulmonary artery arose from the right ventricle (RV) while the Gsk3a -/- showed a normal pattern of development - the aorta coming from the left ventricle (LV) and the pulmonary artery from the right one. In addition the intraventricular septum closed completely in the Gsk3a mutant while in Gsk3b-/- embryos ventricular septal defects (VSD) were commonly observed. Figure 1 demonstrates these changes on tissue level of heart of GSK3b -/-.
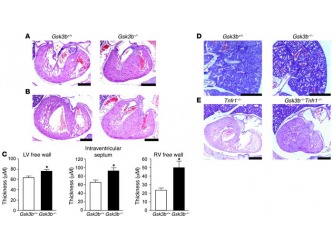
Another noticeable difference was the considerable thicker walls of the LV and RV in the Gsk3b-/- embryos in comparison with the WT (Figure 2 A-C) and the narrower ventricular cavities that arose as a result in these mutants. The figure also compares (Figure 2, D-E) the effect on the ventricular walls and ventricular cavity in mice deficient for both TNF-a receptor and Gsk3b with a control group lacking only the TNF receptor. The data demonstrates that the thickness of the ventricular walls is noticeable bigger in the double mutant embryos than in the control group.
Figure 1Histological analysis of heart development in GSK-3β–deficient mice. Sequential rostral-to-caudal sections from an E15.5 Gsk3b–/– embryo from the pulmonary valve (top left) to the ventricles (bottom right) (original magnification, ×2.0; scale bar: 0.5 cm). The pulmonary valve and the pulmonary artery (P) are positioned over the RV (A–C). Below the pulmonary valve, the aorta (A) descends toward the aortic valve (D–F); the aortic valve is positioned over the RV (G). Caudal to the semilunar valves, the RV and the LV are connected by a VSD (H–K). The location of the VSD is in the membranous portion of the interventricular septum.
.
Comments: I found this additional comparison of the double mutant embryos very interesting. The authors comment on previous studies[18] which reported that Gsk3b deficient organisms showed higher sensitivity to TNF receptor. By conducting such comparative analysis they clearly demonstrate that even without the presence of the receptor, changes of the ventricular walls (more specifically their thickening) is still observed. Thus I can agree with their conclusion that the effect of Gsk3b seems to be independent from the TNF signaling.
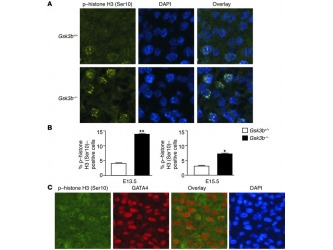
Logically the next question that needs to be addressed is the reason for this phenotype: is the thickening of the ventricular wall result of a) cellular hypertrophy (increase of the average size of the cells), b) reduced apoptosis (programmed cell death) or c) increased proliferation (increase in the number of the myocytes).The researchers were able to eliminate the first two as a reason and supplementary figures 3 and 4 clearly demonstrate their findings. In addition they established that the H3 phosphorylation was increased in myocytes of the Gsk3b-/- in comparison with the WT embryos which is an indication of increase of the proliferation rate (figure 3A and B), further more they prove that the proliferation is due to the cardiomyocytes and not a different type of cells (by demonstrating that more than 90% of the cells that showed positive H3 phosphorylation staining also tested positive for GATA4 expression Figure 3C).
Figure 3
Increased cardiomyocyte proliferation in GSK-3β–deficient hearts. (A) Immunofluorescence staining for nuclear phospho–histone H3 (Ser10) (left panels) in GSK-3β– deficient (bottom) compared with WT (top) animals. Merging of the histone and the DAPI (middle) stains confirmed nuclear localization of the phospho–histone H3 (Ser10) staining (right panels). Original magnification, ×40. (B) Composite mean ± SEM percentage of phospho–histone H3 (Ser10)–staining cells at E13.5 and E15.5 in the myocardium of WT (white bars) and GSK-3β–deficient (back bars) animals.*P < 0.05, **P < 0.001 versus WT. (C) Coimmunostaining of heart sections with phospho–histone H3 and GATA4, a cardiomyocyte marker, demonstrating colocalization in the overlay. Thus, the phospho–histone H3 (Ser10)–positive cells in the myocardium are overwhelmingly GATA4 positive, consistent with cardiomyocytes. Also shown is nuclear staining with DAPI. Original magnification, ×40.
Kerkela,R. (2008 November). Deletion of GSK-3B in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast heperproliferation. Nature. 118, 3609-3618.
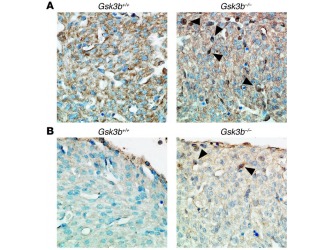
Figure 4
Altered transcription factor and cell cycle regulator expression and localization in GSK-3β–deficient hearts. (A) Increased nuclear cyclin D1 in hearts of E15.5 GSK-3β–deficient embryos. Sections were stained with anti–cyclin D1 antibody (brown) and then counterstained with hematoxylin to identify nuclei (blue). Note the multiple dark brown nuclei (arrowheads) and the overall increase in cyclin D1 stain and corresponding decrease in intensity of the hematoxylin stain in the GSK-3β– deficient hearts. Original magnification, ×40. (B) c-Myc expression is increased in GSK-3β–deficient hearts. Sections from E15.5 embryos were stained with anti–c-Myc antibody (brown) and then counterstained with hematoxylin as described above. Note the enhanced brown staining of the nuclei (arrowheads) and the reduction in intensity of the hematoxylin stain, consistent with increases in nuclear c-Myc expression in the GSK-3β–deficient heart. Original magnification, ×40.
Kerkela,R. (2008 November). Deletion of GSK-3B in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast heperproliferation. Nature. 118, 3609-3618.
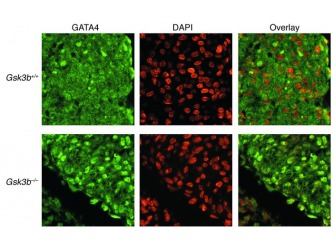
The last step of the research was designed to determine the molecular mechanisms by which Gsk3b regulates cardiomyocytes proliferation and additional genes involved in the process. The molecules in question that were tested included: a) localization of beta-catenin (Supplementary figure 5), b) expression of cyclin D1 (Figure 4A), c) expression of cyclin E (data not shown by the authors), d) expression of cMyc (Figure 4B) and e) expression of GATA 4 (Figure 5 and 6). The investigation determined that a) the distribution of the b-catenin was similar in both WT and gsk3b-/- genotype with no significant differences; b) there is increase in cyclin D1 expression in mutant embryos compared to the WT; c) no differences in expression of cyclin E were observed between the two genotypes; d) there was an increase in nuclear c-Myc expression in the mutant line and e) increase in nuclear GATA4 expression in Gsk3b-/- in comparison to Gsk3b+/+.
The authors conclude that the upregulation of three genes - cyclin D1, c-Myc and GATA4 as a result of Gsk3b loss of function mutation is also essential for the development of the hypertrophic phenotypes observed. Altogether the scientists conclude that Gsk3b is a key regulator in myocyte proliferation by affecting the expression pattern of two transcription factors (GATA4 and c-Myc) and one cell cycle regulator (cyclin-D1). They contribute the phenotypic changes observed in the mutant embryos as a secondary effect due to the lack of regulation from Gsk3b but also suggest that the kinase might play a more direct role in the development of this phenotype. The authors also believe that due the results of their finding application of Gsk3 inhibitors should be carefully considered as therapeutic agents for women in childbearing age.
Discussion: This paper provides an in-depth comparative analysis of the morphological changes that can be observed in heart development in organisms lacking the glycogen synthase kinase and reveal undoubtful evidence that Gsk3b is a key regulator in this process. After their finding that GATA4 is also part of the mechanism that leads to myocyte proliferation the question that first arose was why there were no observed changes in the level of gene expression in the ES cell lines but its levels were elevated in the organismal studies. The authors were able to provide a very plausible explanation for the fact that GATA4 expression is regulated by the beta-catenin pathway in early embryological development and the reason that the b-catenin levels and localizations are not affected is due to the redundant regulatory role of the Gsk3a. It was interesting to observe the gradual and logical outline of their experiment which addresses the changes observed on tissue level, embryonic level an even try to suggest the molecular relationships between the genes in question. Even though the evidence that the scientists provide does in fact conclude the importance of Gsk3b in the developmental processes the exact molecular mechanisms of interaction remains unclear.
Their experiment regards the condition where the whole protein is missing which subsequently removes all of its domains and interactions. In addition the paper suggests that Gsk3a plays a redundant role in beta-catenin pathway which may become an interesting question for research - where is this redundancy expressed and how common are the inhibitors for the two kinases.
These are the type of questions I will try to address in my further project research where I will examine the available data about the structure of the enzyme and try to provide a plausible molecular mechanism to explain its function and the way it is regulated.
Figure 5 Increased nuclear GATA4 expression in GSK-3β–deficient hearts. GATA4 staining (left) was located over nuclei in the hearts of E13.5 embryos stained by DAPI (center) in merged images (right). Original magnification, ×40.
Kerkela,R. (2008 November). Deletion of GSK-3B in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast heperproliferation. Nature. 118, 3609-3618.
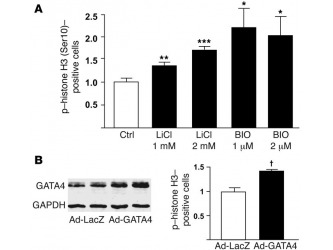
Figure 6 GATA4 overexpression drives cardiomyocyte proliferation. (A) GSK-3
inhibition increases neonatal rat ventricular myocyte (NRVM) proliferation. Shown is the mean ± SEM percentage of phospho–histone H3 (Ser10)–staining cells 20 hours following LiCl and 6-bromoindirubin- 3ʹ-oxime (BIO) treatment at the indicated concentrations. (B) GATA4 overexpression induces NRVM proliferation. Immunoblot of lysates from NRVMs infected with adenovirus encoding LacZ (left 2 lanes) or GATA4 (right 2 lanes). NRVMs were treated with 100 viral particles per cell (first and third lanes) or 200 particles per cell (second and fourth lanes). NRVMs plated onto glass coverslips were stained for phospho–histone H3 (Ser10) 48 hours after infection of cells with adenoviruses (200 viral particles per cell) encoding GATA4 (Ad-GATA4) or LacZ (Ad-LacZ). Shown is the mean ± SEM percentage of phospho–histone H3 (Ser10)–staining cells following Ad-LacZ or Ad-GATA4 infection. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle control (Ctrl); †P < 0.001 versus Ad-LacZ.
Kerkela,R. (2008 November). Deletion of GSK-3B in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast heperproliferation. Nature. 118, 3609-3618.
Scientific vs Primary article review.
The popular press article is able to capture the main findings in the research. They introduce the fact that Gsk3b has a role in the heart development and raise the question of possible negative effects Gsk3 inhibitors may have if used as therapeutic agents in women in childbearing age. It is able to introduce the information in a very concise manner without going into unnecessary details of how the results were obtained. I personally find this article to accomplish the common goal – it informs the public for a recent discovery in the genetics and provide a summary of its physiological significance. At the same time the information provided by the magazine give further details of where one can find the specifics of the research experiments conducted. The research article on the other hand provides detailed mechanisms of the different steps and experiments conducted by the researchers. The scientists are able to express their specific goals very clearly and provide clear and well based analysis of their findings. Even when the question that they addressed gave some ambiguous results they were able to provide plausible explanations of why it had happened.
Back to Home page
References:
3.Science daily (2008, October 3). Loss of the protein target of lithium disrupts normal mouse embryonic heart development. Retrieved February 1, 2009 from http://www.sciencedaily.com
8.Development of GSK-3 inhibitors by GlaxoSmithKline and by Johnson & Johnson. (2003, September 17) Bioportfolio Retrieved February 7, 2009 from http://www.bioportfolio.com
2. Kerkela,R. (2008 November). Deletion of GSK-3B in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast heperproliferation. Nature. 118, 3609-3618.
16. Cohen, L.S., Friedman, J.M. (1994). A reevaluation of risk of in utero exposure to lithium. JAMA. 271:146-150
17. Schulte-Merker, S. & Smith, J.C. (1995). Mesoderm formation in response to brachyury requires FGF signaling. Current biology. 5:62-67
18. Hoeflich, K. (2000) Requirement for glycogen synthase kinase-3 beta in cell survival and NF-kapaB activation. Nature. 406:86-90
Contact information
This webpage was created by: Eva Dimitrova, [email protected]